
At Penman Consulting, we have been providing IUCLID training in various forms since 2008. I sat down with one of our Toxicologists, Jamie Dunn MSc. to see if he could give us any tips. Jamie uses IUCLID daily and often, like other colleagues, runs training sessions; external presentations, front-end IUCLID support and in-house training for our new starters, like Laia and Ema. Jamie has also provided training to our clients’ employees within the chemical and petrochemical sectors.
“It’s important to understand the structure of IUCLID. Depending on the substance, different companies and consortia will have different arrangements. Knowing how templates and other items are set up is important. Here is an anonymised example of one of the dossiers we manage.
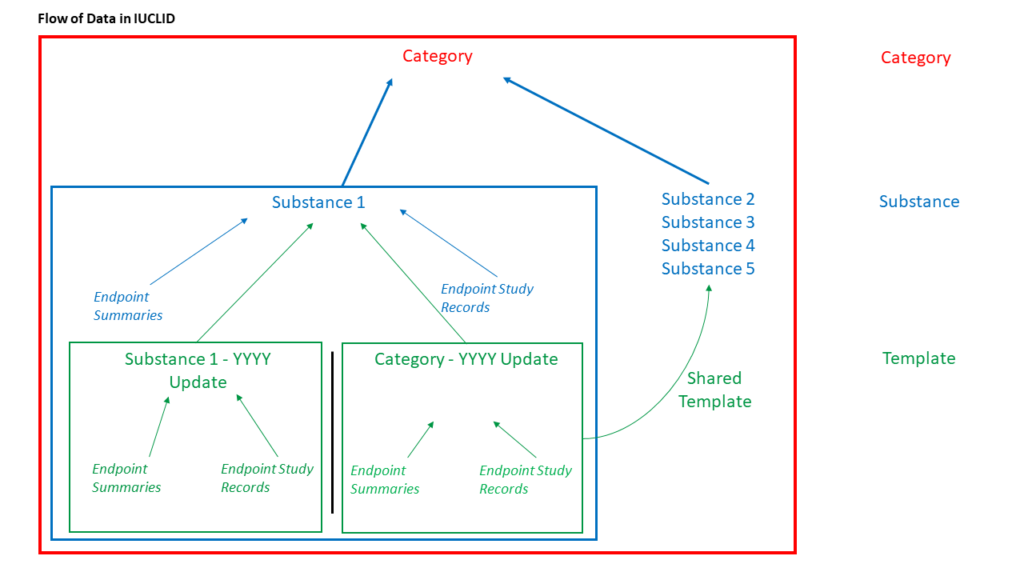
IUCLID is designed so that you don’t have to send lots of studies to the regulators. The idea is you can present scientific arguments in a concise and structured way, and it allows the regulators to evaluate your key decisions quickly and effectively. It is designed to be used in place of sending full study reports.
When you are writing study summaries it is important to focus on the key information and not the minutiae of studies. To evaluate the information and create conclusions, rather than just repeating all of the data.”
“Well, the top of my wish list is colour! IUCLID is very grey!
But on a more serious note, I would like a better change log and the ability to tell what has changed between modifications. We can and do take backups, but it would be useful to know what has changed in detail. Similarly, the ability to undo mistakes would also be nice and it is particularly important for those in training! Internally at Penman Consulting we have training instances, so users can get comfortable working in IUCLID without overwriting live data.
We are also trialling the web interface, and although that is still in development it would be good to see some improvements. For example, currently it doesn’t have a tree of contents and that is the easiest way to navigate.”
“Well, IUCLID is used for more than just REACh, Classification, Labelling and Packaging regulations (CLP) and Biocidal Product Regulation (BPR). I know since I started using it, it has been adapted for New Zealand and Australian regulations, so learning more about IUCLID is always a useful skill to have, it is now applicable in many areas!
There are many different ways of presenting the same data, and I guess this ties into the question on structure. If you know how, there are ways that you can cut down the time spent entering study records by half, or even more. Templates are your friend and can be used for multiple purposes, rather than just to hold a whole data set. They can be used as vehicles to exchange information.
Unfortunately there is no spell check in IUCLID, I always make a point of emphasising this during training sessions. Occasionally there are system spelling errors which cannot be “pevented” by the user (P243, relating to static discharges).”
To learn more, please email info@penmanconsulting.com. We can provide general and specialised IUCLID training for your organisation.