
Once an IUCLID dossier has been completed, the final step is for the registrant to submit the dossier to ECHA. This is done through the platform “REACH-IT” where registrants submit all information to ECHA (such as registrations, inquiries and PPORD notifications).
I spoke to James Orr from our PRSA (Product Stewardship and Regulatory Affairs) team about submitting dossiers to ECHA. He manages over 60 registrations as our Only Representative Manager.
The main three pre-requisites are:
“In IUCLID, right-click on the file and then select “Create Dossier”. You then get taken through a wizard, where you can fill in different information about the dossier. Firstly, you need to know the type of dossier, you can choose between lead registrant/member registrant and then different tonnage options.
You will also need to identify whether the Guidance on safe use and CSR (Chemical Safety Report) are being provided by the lead registrant. This information can be found in the joint submission on REACH-IT. A lead registrant may sometimes provide a CSR even if it is not marked as “Provided by lead” in REACH-IT. This just means that the overall responsibility for the CSR is with the registrant.”

“You also need to select if the dossier is an update (most of the time this is the case) and if so, you will need the last submission number – which is also found in REACH-IT. You also need to provide the reason for the update. If it is a spontaneous update then you must give a justification, such as a change of composition, updated tonnages, or an updated CSR.
Once you have exported the dossier from IUCLID, you then need to submit it in REACH-IT. Once you are logged into REACH-IT, to submit you can either select “Submit a dosser” on the main menu or navigate through the joint submission.
When you submit a dossier there are several processing steps for the submission. If one of these fails REACH-IT will give you information about why the submission has failed.”
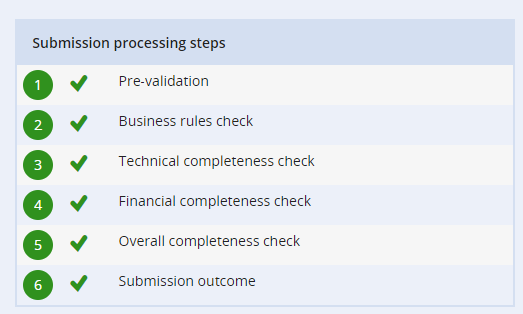
“I think it is important to use the tools available in IUCLID, like the Validation Assistant Tool (VAT check). This can catch many reasons why the dossier might be rejected automatically due to missing information. However, it does not catch all mistakes involved in the creation of the dossier, such as an incorrect previous submission number.
Before you can submit the registration, make sure that you are part of the “Joint Submission”. A small tip for when you are trying to join this, make sure you select the correct “Join Existing” option from the menu. Recently ECHA added a new section to the bottom of the menu “Joint application for authorisation”. As they have similar names and are in the same place, it is easy to click by default on the wrong “Join Existing” option, especially if that is what you are used to!”
If you’re interested in how Penman Consulting can help you with your regulatory submissions, please contact us at info@penmanconsulting.com.